REPORTS
Intensive Glycemic Treatment During Type 1 Diabetes Pregnancy
Presented by:Prof. Helen R. Murphy
Diabetes & Antenatal Care, Norwich Medical School, University of East Anglia, Norwich, UK
Department of Women & Children’s Health, King's College London, London, UK
In 2016 the UK National Pregnancy Database collected data from approximately 3,300 women with diabetes (type 1 diabetes 1,618; type 2 diabetes 1,608), 3,300 pregnancies, and 3,350 births.1 According to the registry, there has been an approximate 40% increase in type 1 diabetes as women are now being diagnosed earlier and entering pregnancy with a longer duration of diabetes.1 Furthermore, about half of the women with type 1 diabetes are entering pregnancy either overweight or obese.1
There has been a steady reduction in newborn stillbirths from just 10 years ago (2.5-fold reduction according to Confidential Enquiry into Maternal and Child Health (CEMACH; 10.7 vs. 25.8/1,000 T1D p = 0.0012; 10.5 vs. 29.2/1,000 T2D, p = 0.0091).1,2 Unfortunately, of the mothers with type 1 diabetes, <15% achieved target HbA1c goals and there continues to be a lack of improvement in the antenatal glucose control in type 1 diabetes. In fact, the median HbA1c level of both type 1 and type 2 mothers in the first trimester was 58 mmol/mol (48-69) in 2002, and has increased to 61 mmol/mol (45-89) for type 1 and 51 mmol/mol (39-81) in type 2.1 However, there appears to be significant variation from clinic to clinic, with an average of only 14% of type 1 women reaching target HbA1c (≤48 mmol/mol) during the first trimester, but the clinic-to-clinic range was 0 to 44%. The wide range seen shows that many patients do not achieve target at any point.1 The third trimester HbA1c results also show significant variation with 38% (range 0-82%) of type 1 diabetes pregnancies reaching target HbA1c levels.1 These significant failures to reach target HbA1c levels have led to larger for gestational age (LGA) babies (47% in type 1 and 23% in type 2), with 40% of babies from type 1 diabetes mothers admitted to the Neonatal Intensive Care Unit (NICU), 60% preterm (<37 weeks) from type 1 diabetes mothers, and approximately 90% <34 weeks from type 1 diabetes mothers.1
The objective of the CONCEPTT trial was to determine if continuous glucose monitoring (CGM) in pregnant women would change HbA1c at 34 weeks’ gestation favorably. Approximately 100 pregnant women were enrolled in the study.3 The median age was 31.4 years with 87% being of European origin. Approximately 50% had diabetes for 10-20 years with 34% having diabetes for more than 20 years. While the number of patients was relatively small, the CGM showed a statistically significant difference of -0.2% in HbA1c levels (95% CI, -0.34, -0.03; p = 0.0207). It is important to note that these findings were consistent across sites and methods of insulin delivery (pumps, pen injections). An added consideration to note is that, due to increased red cell turnover early in pregnancy, the HbA1c levels decreased and were unrelated to glucose levels.3
As fetal glucose exposure is evaluable with CGM in pregnant women, a review was conducted to explore the amount of time pregnant women reached/remained at target glucose levels (3.5-7.8 mmol/L [63-140 mg/dL]) through pregnancy. Of 214 women on CGM (n = 107) and control (n = 107), 52% of the time (12.5 hours/day) was spent at target glucose levels during the first trimester. At 34 weeks’ gestation, 68% of time (16.3 hours/day) was maintained at target glucose levels in women on CGM compared to 61% of time (14.6 hours/day) for the control group (p = 0.0034).4 The amount of time in hyperglycemic (>7.8 mmol/L (>140 mg/dL) was 39% (9.6 hours/day) in the CGM at baseline and 27% (6.5 hours/day) at 34 weeks’ gestation compared to the control group: 40% (9.6 hours day) and 32% (7.7 hours/day), baseline and 34 weeks respectively.4 There was a reduction in the amount of time spent in hypoglycemia (<3.5 mmol/L [63 mg/dL]) with 8% of women on CGM at baseline and 3% of women at week 34 compared to control (6% and 4%, respectively).4 Data were not statistically significant because of the small amount of participants experiencing hypoglycemia in this study.
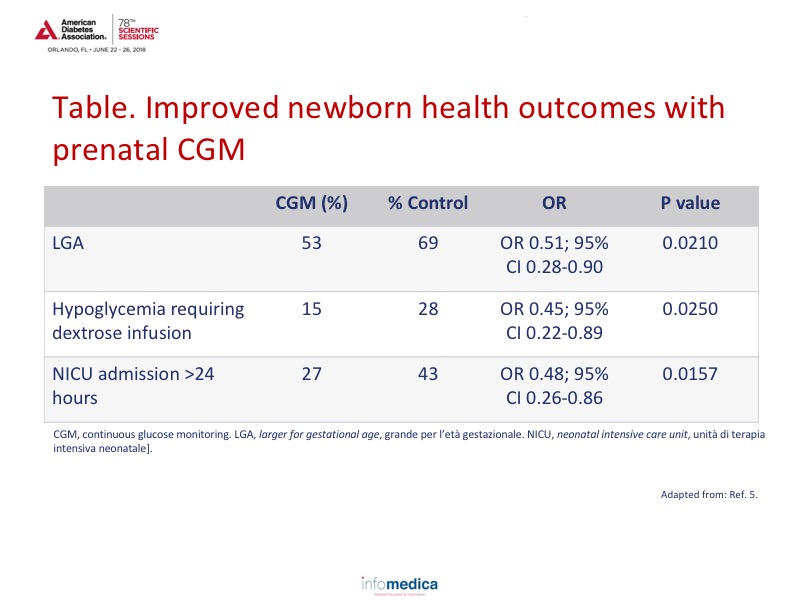
Newborn health outcomes also improved with CGM. See Table for LGA, hypoglycemia, and NICU admission data.5 In addition, the median length of stay for infants was also decreased in the CGM group with 3.1 (2.1-5.7) days in the CGM group compared to 4.0 (2.4-7.0) in the control group (p = 0.0157).
There is limited information for closed-loop (automated) insulin delivery vs. sensor-augmented pump (SAP) therapy in pregnant women. To learn more about the ability of maintaining glucose targets consistently during pregnancy, a 4-week, randomized, crossover study using an overnight closed-loop system was completed. The primary efficacy endpoint was the time with glucose in the target range (63-140 mg/dL) overnight, as recorded by CGM.5 The percentage of overnight time that glucose values were within target range was significantly higher with closed-loop therapy than with SAP therapy (74.7% vs. 59.5%; absolute difference, 15.2 percentage points; 95% CI, 6.1-24.4, p = 0.002).5 Mean glucose levels were significantly lower during closed-loop therapy than during SAP therapy, both overnight (119 vs. 133 mg/dL [6.6 vs. 7.4 mmol/L], p = 0.009) and over a 24-hour period (128 vs. 137 mg/ dL [7.1 vs. 7.6 mmol/L], p<0.001).5
Key Messages
- Women are being diagnosed with type 1 diabetes earlier and thus enter pregnancy with a longer duration of diabetes.
- Many women with diabetes are entering pregnancy either overweight or obese.
- Babies born to mothers with diabetes type 1 are prone to LGA, preterm deliveries, and NICU admissions.
- CGM in pregnant women has shown favorable results for maintaining target glucose levels.
- Patients using CGM had a higher percentage of time at target glucose levels and a lower amount of time at hyperglycemic levels compared to SAP.
REFERENCES
Present disclosure: The presenter has reported that she supports research for Medtronic and Abbott Diabetes Care, and is a speaker for Medtronic, Abbott Diabetes Care, and Novo Nordisk.
Written by: Debbie Anderson, PhD
Reviewed by: Marco Gallo, MD