会议要点
基础胰岛素后的升级治疗:GLP-1激动剂 vs. 餐时胰岛素vs.预混胰岛素
作者Helena W. Rodbard, MD, FACP, MACE
Medical Director, Endocrine and Metabolic Consultants
Rockville, MD; USA
2型糖尿病患者需关注临床惯性的问题,因为血糖控制不佳与糖尿病并发症有明确的相关性。临床惯性的一个常见问题是不能及时对基础胰岛素治疗进行升级或强化1。在接受基础胰岛素治疗的患者中,HbA1c≥7.5%的患者只有30.9%接受了强化治疗,只有20.9%的患者在6个月内HbA1c降到了7.5%以下1。
根据ADA的推荐,强化方案的调整包括:GLP-1受体激动剂、胰岛素和预混胰岛素2。以下将对各项方案进行详细分析。
GLP-1受体激动剂的药代动力学作用不一,既有长效也有短效。短效药物有艾塞那肽bid,利司那肽qd,利拉鲁肽qd,半衰期分别为2.4h、3h和13h3。长效药物有杜拉鲁肽qw,艾塞那肽qw和索玛鲁肽qw,半衰期分别为~4天,7~14天和~7天。
一项研究表明,在血糖控制不佳的2型糖尿病患者中,基础胰岛素联用利司那肽可有额外获益4。试验组平均HbA1c水平下降至7.8%,而对照组为8.1%,且体重也有改善(-1.3kg vs -0.01kg)。同时,胰岛素用量也有所下降(50U vs. 57U)。与预期相符,短效GLP-1受体激动剂降空腹血糖的作用不显著 4。但在7时点血糖谱(早餐前,早餐后2小时,午餐前,午餐后2小时,晚餐前,晚餐后2小时,睡前)中,利司那肽组的血糖控制比对照更佳。
AWARD-9研究则对比了杜拉鲁肽1.4mg/w+甘精胰岛素qd vs. 安慰剂+甘精胰岛素qd的疗效差异5。纳入标准为已使用基础胰岛素±二甲双胍且HbA1c水平在7.0%-10.5%5。结果显示,杜拉鲁肽组的HbA1c下降幅度显著大于安慰剂组(-1.44% vs. -0.67%,p <0.001);体重也较安慰剂组明显下降(-1.91 kg vs. +0.50 kg,p <0.001))。
近期发表的SUSTAIN 5研究则观察了基础胰岛素联合索马鲁肽对2型糖尿病患者的疗效6。纳入标准为HbA1c水平在7.0%-10%且已使用基础胰岛素±二甲双胍规律治疗的患者,样本量近400例。此研究使用了两种剂量的索马鲁肽(0.5mg和1mg)。两种剂量的索马鲁肽相比安慰剂组均显著改善了HbA1c水平(索马鲁肽0.5mg、1.0mg和安慰剂组分别使HbA1c降低1.3%、1.7%和0.2%,p<0.001)。体重也呈显著性下降(7.7lbs、13.2lbs和2.6lbs)。
LixiLan-L试验则表明,与单用IGlar相比,2型糖尿病患者使用剂量可调的固定配比甘精胰岛素(IGlar)+利司那肽复方制剂,根据血糖调整剂量,可更好地实现HbA1c达标及减重7。另外,使用复方制剂的患者低血糖事件的发生率和年事件数也低于单用甘精胰岛素。
还有一项研究则在二甲双胍+基础胰岛素控制不佳的2型糖尿病患者中,比较利拉鲁肽qd+甘精胰岛素(IdegLira)vs.基础-餐时胰岛素的疗效和安全性8。逾500名患者被纳入该试验,随机分为IDegLira+二甲双胍组和IGlar +门冬胰岛素(IAsp)+二甲双胍组。纳入标准为HbA1c在7.0%-10.0%。结果显示,IDegLira组在HbA1c <7%且未增重,HbA1c <7%且未发生低血糖,HbA1c <7%未发生低血糖且未增重的病人比例均显著高于胰岛素组。但就单纯HbA1c <7%的患者比例,两组无显著差异8。
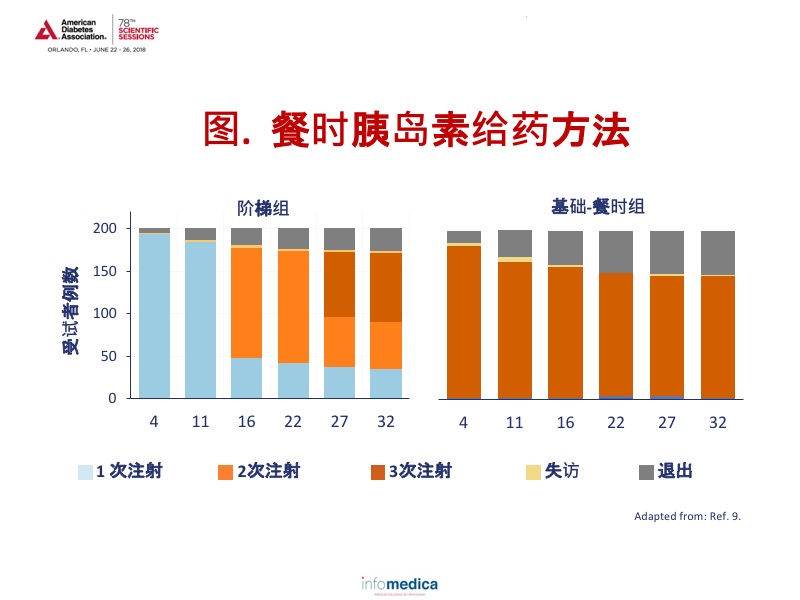
FullSTEP研究的目的是确定当2型糖尿病患者需要使用餐时胰岛素时,在最大一次进餐的餐前给予IAsp,其效果是否与使用三次餐前IAsp一样有效。9纳入标准为:2型糖尿病病程至少12个月,使用基础胰岛素至少6个月,HbA1c在7.0%-9.0%。血糖控制目标为:空腹血糖71-130 mg/dL且HbA1c <7%。患者根据HbA1c水平按7.0%-8.0%和8.1%-9.0%分层。基础-餐时组的患者在三餐前均接受IAsp治疗。阶梯组在最大的一次进餐前注射IAsp;如果HbA1c维持在7%或更高,于第11周和第22周分别在次大餐前给予IAsp。在33周的时间内,阶梯组的平均HbA1c从7.9%降至6.9%,而基础-餐时组从7.9%降至6.8%(p=0.088)9。值得注意的是,基础-餐时组退出人数是阶梯组的两倍,显示出更好的耐受性和满意度。9见图示。
与基础-餐时组相比,阶梯组的低血糖发生率较低,包括总事件率、有症状、无症状、ADA标准未分类的和夜间发作的低血糖,比例约为2:1。
一项临床试验在先前使用基础胰岛素-IDeg和IAsp治疗的2型糖尿病患者中,以确定两种胰岛素强化策略(每日两次预混胰岛素或基础-餐时分次注射给药)的疗效和安全性10。结果显示,IDegAsp与IDeg + IAsp相比,HbA1c降低或低血糖发生率无统计学意义。10
关键信息
- 即使在患者血糖高于目标水平时,也常常存在胰岛素加强治疗的延迟。
- GLP-1受体激动剂包括短效或长效药物,与胰岛素治疗联用均有将血糖降低到目标水平、降低体重和减少低血糖风险的效果。
- 与基础-餐时方案相比,阶梯法使用餐时胰岛素同样可降低HbA1c并减少低血糖风险。
- 预混胰岛素方案与基础-餐时胰岛素相比,HbA1c下降幅度和低血糖发生率无显著性差异。
参考文献
Present disclosure: The presenter reported that she was a consultant for Bayer, BI/Lilly, Janssen, Lexicon, Merck, Novo Nordisk, Sanofi, and Regeneron. She is also a speaker for Astra Zeneca, BI/Lilly, Merck, Novo Nordisk, and Sanofi and she provides research support for Astra Zeneca, BI/Lilly, Janssen, Lexicon, Merck, Mylan, Novo Nordisk, Sanofi, and Regeneron.
Written by: Debbie Anderson, PhD
Reviewed by: Marco Gallo, MD