REPORTS
Therapy Progression After Basal Insulin: GLP-1 Agonists vs. Prandial Insulin vs. Premixed Insulin
Presented by:Helena W. Rodbard, MD, FACP, MACE
Medical Director, Endocrine and Metabolic Consultants
Rockville, MD; USA
Clinical inertia is a concern in patients with type 2 diabetes because there is a clear correlation between poorly controlled blood glucose levels and diabetes complications. A critical step in clinical inertia is the frequent delay between initiation of basal insulin and treatment intensification.1 Among patients who have been placed on basal insulin, only 30.9% of patients whose HbA1c increased to ≥7.5% received treatment intensification and only 20.9% reached HbA1c levels of <7.5% in 6 months.1
Options for intensifying treatment, based on the ADA algorithm, include GLP-1 RAs, insulin, and premixed insulin.2 We will review each of these in more detail.
The pharmacokinetics of GLP-1 RAs vary as they can be either short- or long-acting. The short acting agents are exenatide BID, lixenatide QD, and Liraglutide QD with half-lives of 2.4 hours, 3 hours, and 13 hours respectively.3 The longer acting agents include dulaglutide QW, exenatide QW, and semaglutide QW with half-lives of ~ 4 days, 7-14 days, and ~7 days respectively.3
Added benefits were demonstrated in a study that added lixisenatide to basal insulin in type 2 diabetes patients with inadequately controlled diabetes.4 The mean HbA1c level improved to 7.8% compared to control 8.1%, as did the mean change in body weight (-1.3 kg vs.-0.01 kg). There was also a reduction in mean insulin units (50 vs 57).4 Changes in fasting plasma glucose were not evident nor expected with a short-acting GLP-1 RA.4 There was also an improvement in the 7-point glucose profile (pre-breakfast, 2-hour post-breakfast, pre-lunch, 2-hour post-lunch, pre-dinner, 2-hour post dinner, and bedtime) in the lixisenatide group compared to placebo.4
The AWARD-9 study explored the use of dulaglutide 1.4 mg once weekly + insulin glargine (IGlar) once daily vs. placebo + IGlar in patients with type 2 diabetes.5 Patients had to remain on basal insulin ± metformin and have HbA1c levels ≥7.0% to ≤10.5% to be included in the study.5 The dulaglutide group demonstrated a statistically significant reduction in HbA1c of -1.44% vs. -0.67% with placebo p <0.001), as well as a reduction in body weight -1.91 kg vs. +0.50 kg with placebo (p <0.001).5
The recently published SUSTAIN 5 study examined semaglutide when added to basal insulin in patients with type 2 diabetes.6 Just under 400 patients with HbA1c levels between 7.0% and 10% on stable treatment with basal insulin alone or in combination with metformin were included in the study.6 Two doses of semaglutide were studied, 0.5 mg and 1 mg.6 Statistical significance was achieved in the mean change in HbA1c from baseline in both the semaglutide 0.5 mg and 1.0 mg doses (-1.3 and -1.7% respectively, p <0.001) compared to placebo (-0.2).6 There was also a reduction in the mean body weight change from baseline with both doses of semaglutide compared with placebo (-7.7 lbs, -13.2 lbs, -2.6 lbs, semaglutide 0.5 mg, 1.0 mg, and placebo respectively).6
In the LixiLan-L trial, a titratable, fixed-ratio combination of IGlar and lixisenatide demonstrated improvements in HbA1c reduction, patients at target HbA1c, and weight change in patients with type 2 diabetes compared with patients treated with IGlar alone.7 Patients on the lixisenatide combination also demonstrated a lower percent of hypoglycemia events and number of events per year compared to IGlar alone.7
The last study discussed was a trial exploring the efficacy and safety of liraglutide once daily + insulin glargine (IdegLira) vs. basal-bolus insulin therapy in patients with type 2 diabetes who were uncontrolled on metformin and basal insulin.8 More than 500 patients were randomized to receive either IDegLira + metformin or IGlar + insulin aspart (IAsp) + metformin. To qualify for the study, patients needed to have an HbA1c between 7.0% and 10.0%.8 Statistical significance was seen in the proportion of patients on IDegLira who achieved an HbA1c of <7% without weight gain, those who achieved an HbA1c of <7% with no hypoglycemia, and those who achieved an HbA1c of <7% with no hypoglycemia and no weight gain compared to the IGlar group.8 Statistical significance was not achieved in the number of patients who achieved an HbA1c of <7% alone.8
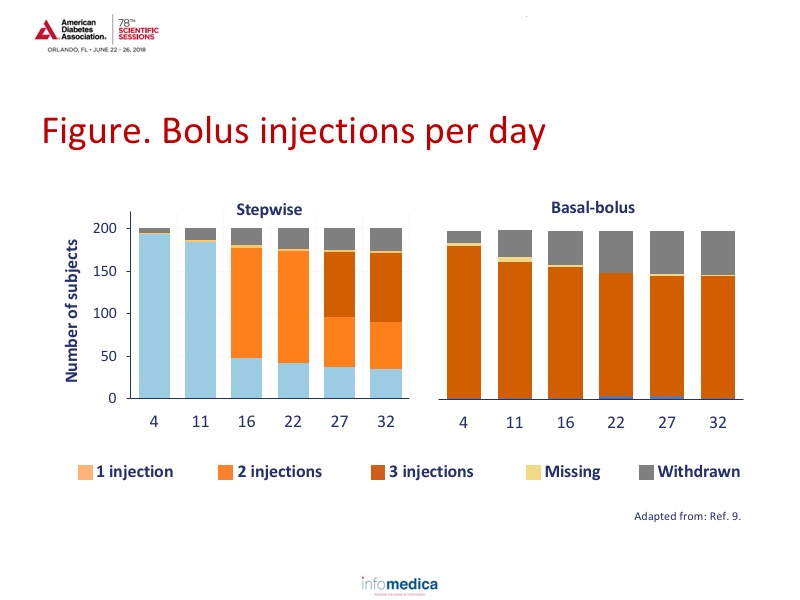
The FullSTEP study was designed to determine if step-wise addition of IAsp (1 mealtime insulin before the largest meal) was as effective as initiating with 3 bolus doses, when intensification with bolus insulin was needed.9 To be included in the study, patients needed to have diabetes type 2 for a least 12 months, be taking basal insulin for at least 6 months, and have an HbA1c of 7.0% to 9.0%.9 Glycemic target goals were identified as fasting plasma glucose of 71-130 mg/dL and HbA1c of <7%.9 Patients were stratified according to HbA1c intervals of 7.0%-8.0% and 8.1%-9.0%.9 Patients in the basal-bolus group received IAsp before every meal. Patients in the stepwise group received 1 bolus dose with the largest meal with additional IAsp doses before the next largest meal at 11 weeks and 22 weeks if the HbA1c remained at 7% or higher. Over a 33-week period, the mean HbA1c reduced in the stepwise group from 7.9% to 6.9% while the basal bolus group decreased from 7.9% to 6.8% (p = 0.088).9 Notably, there were twice as many withdrawals in the basal-bolus group compared to the stepwise group demonstrating greater tolerability and enhanced patient satisfaction.9 See Figure.
Patients in the stepwise approach also had reduced rates of hypoglycemia compared to the basal bolus treatment for all episodes, documented symptomatic, asymptomatic, ADA unclassifiable, and nocturnal episodes, many at a relative 2:1 ratio.9
A clinical trial was completed to determine the efficacy and safety of two insulin intensification strategies for patients with type 2 diabetes previously treated with basal insulin—IDeg and IAsp when administered as a coformulation twice-daily or as a basal-bolus regimen (separate injections).10 There was no statistical significance in HbA1c reduction or rates of hypoglycemia when comparing IDegAsp vs. IDeg + IAsp.10
Key messages
- There is often a delay in insulin intensification even when patients are above target glycemic levels.
- GLP-1 RAs can either be short- or long-acting and have demonstrated efficacy in reducing HbA1c to target levels, reducing weight, and reducing risk of hypoglycemia when used in combination with insulin therapy.
- The progressive addition of rapid acting insulin boluses reduces HbA1c and reduces risk of hypoglycemia relative to a basal bolus regimen.
- Regimens with premixed insulin reduce HbA1c and the rate of hypoglycemia as much as a basal-bolus regimen.
REFERENCES
Present disclosure: The presenter reported that she was a consultant for Bayer, BI/Lilly, Janssen, Lexicon, Merck, Novo Nordisk, Sanofi, and Regeneron. She is also a speaker for Astra Zeneca, BI/Lilly, Merck, Novo Nordisk, and Sanofi and she provides research support for Astra Zeneca, BI/Lilly, Janssen, Lexicon, Merck, Mylan, Novo Nordisk, Sanofi, and Regeneron.
Written by: Debbie Anderson, PhD
Reviewed by: Marco Gallo, MD